Marketing medical products requires balancing regulatory compliance with effective promotion strategies. Healthcare marketers develop messaging that communicates product benefits while adhering to FDA guidelines and industry regulations. Successful medical product marketing includes regulatory review, targeted audience segmentation, clear evidence-based messaging, appropriate channel selection, and ongoing performance measurement to drive adoption while maintaining compliance with healthcare marketing rules.
Understanding Regulatory Requirements
Medical product marketing operates within regulatory frameworks that vary by product type and market. FDA regulations govern what claims manufacturers can make about drugs, devices, and other medical products. Marketing materials require appropriate risk disclosures and fair balance between benefits and potential side effects. Different product classifications face varying promotional restrictions that marketers must know. International markets have their own regulatory bodies with different requirements. Healthcare organizations implement review processes where legal and regulatory teams evaluate all marketing content before publication. This regulatory foundation influences every aspect of medical product marketing strategy.
Defining Target Audiences and Messages
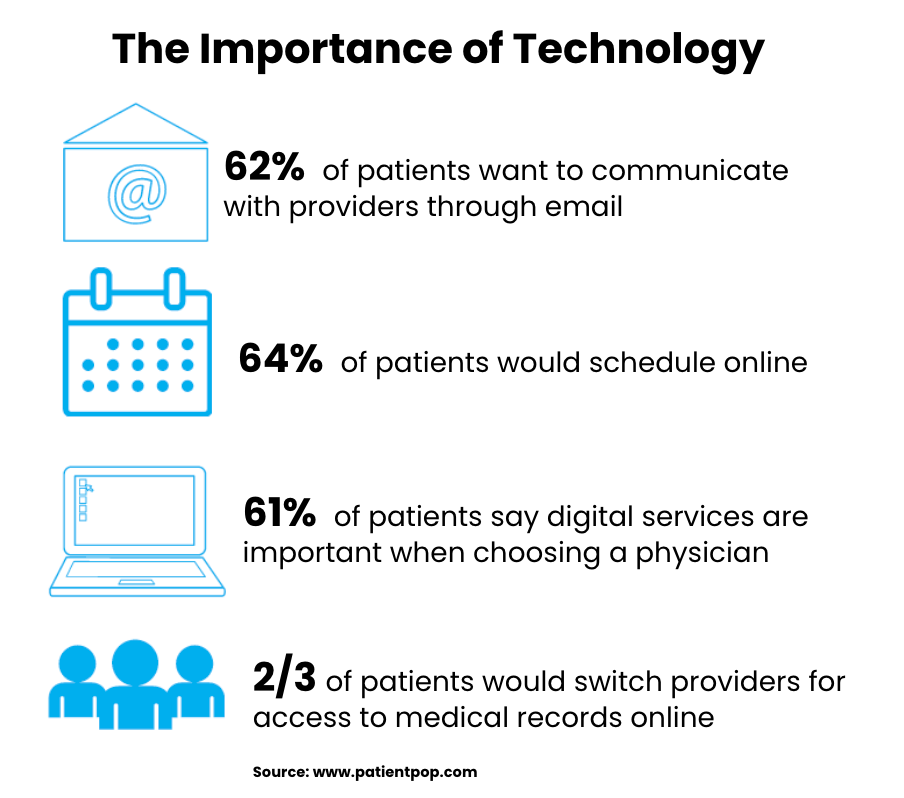
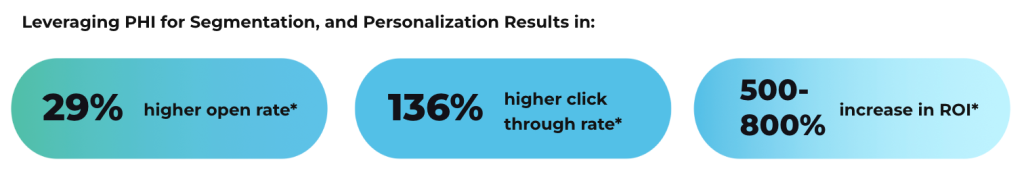
Medical product marketing works best with precise audience segmentation based on who influences purchasing decisions. Campaigns typically target multiple stakeholders including healthcare providers, administrators, payers, and patients. Research reveals each audience’s needs, pain points, and decision factors. Message development addresses how the product solves clinical challenges or improves outcomes for each audience segment. Healthcare providers often respond to technical details and clinical evidence, while patients prefer clear explanations of benefits. Payers concentrate on economic value and comparative effectiveness. Well-crafted messages help various audiences understand how a product relates to their healthcare concerns.
Creating Evidence-Based Marketing
Medical product marketing relies on credible evidence supporting product claims. Clinical studies form the basis for marketing messages about efficacy and safety. Case studies show real-world applications and results. Health economic data helps present the financial case to payers and administrators. Marketing teams collaborate with medical affairs departments to ensure accurate presentation of research findings. Materials distinguish between established facts and emerging evidence. This approach builds credibility with healthcare audiences while adhering to regulatory compliance. Marketing departments document connections between promotional claims and supporting research.
Choosing Marketing Channels
Healthcare audiences respond differently to various communication channels based on how they prefer receiving information. Digital platforms include medical websites, professional networks, email campaigns, and virtual events for healthcare professionals. Print materials and journal advertising reach providers during clinical reading time. Conferences and trade shows allow direct product demonstrations. Patient education materials might include websites, videos, and print resources designed for easy consumer understanding. Marketing teams select channels considering audience media habits, message complexity, and regulatory factors. Using multiple channels often works well by reaching audiences through their preferred information sources.
Developing Sales Force Capabilities
Many medical products depend on sales representatives who talk directly with healthcare providers. These representatives learn both product details and regulatory boundaries for promotional discussions. All sales materials undergo compliance review to ensure appropriate claims. Medical science liaisons often support more technical conversations about research and clinical applications. Companies coordinate marketing campaigns with sales activities to reinforce important messages. Digital engagement now supplements traditional sales visits through virtual meetings and online presentations. This personal contact helps answer questions while developing relationships with healthcare decision-makers.
Evaluating Marketing Results
Medical product marketing needs clear performance metrics connected to business goals. Marketing teams monitor awareness indicators like website visits, material downloads, and event attendance. Engagement measurements track time spent with content, inquiries received, and follow-up requests. Conversion metrics show how marketing influences prescribing behavior, product orders, or contract decisions. Analytics tools help identify which channels and messages generate the best results. These measurements guide refinements to marketing strategies and resource allocation. Performance data demonstrates marketing return on investment to leadership teams.