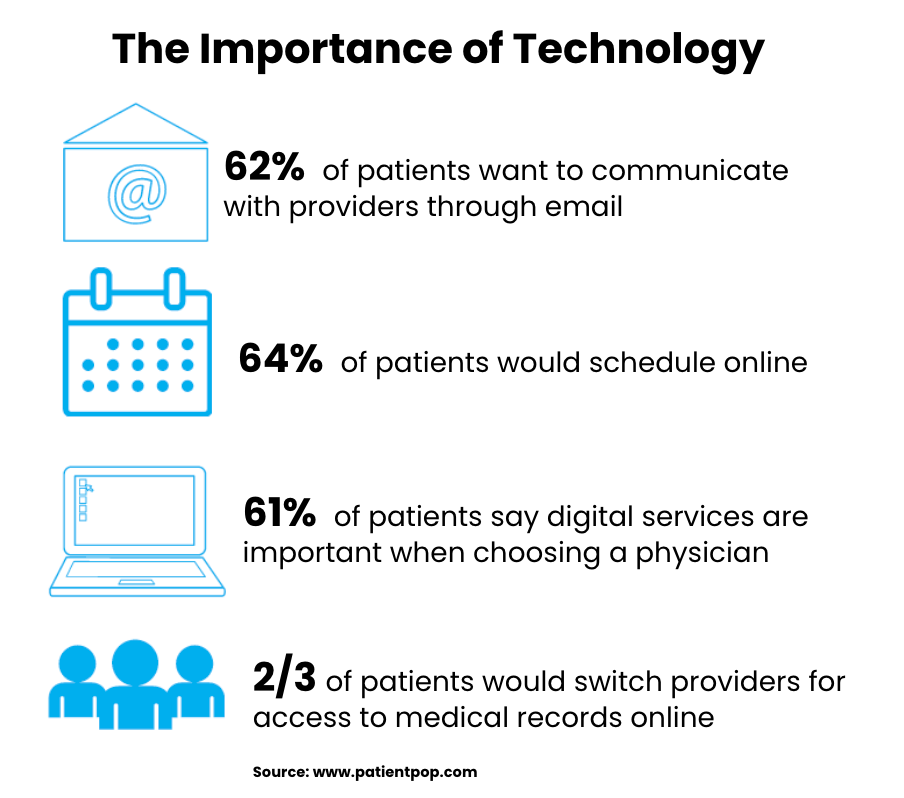
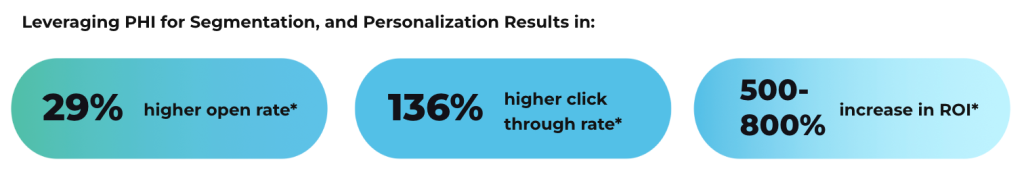
A HIPAA email retention policy should include classification procedures for different email types, retention schedules based on content and legal requirements, secure storage and disposal methods, access controls for archived communications, and compliance monitoring procedures. The policy must address both HIPAA documentation requirements and broader legal obligations while providing clear guidance for staff implementation and ongoing management. Healthcare organizations need comprehensive retention policies that address complex regulatory landscapes without creating unnecessary administrative burden. Well-designed policies help ensure compliance while managing storage costs and supporting operational efficiency across the organization.
Email Classification and Categorization Guidelines
Content-based categories help staff identify appropriate retention periods by distinguishing between patient care communications, administrative messages, and marketing materials. Each category should have clear examples and decision criteria to ensure consistent application. PHI identification procedures enable staff to recognize when email communications contain protected health information requiring special handling and extended retention periods. These procedures should address obvious PHI like patient names as well as indirect identifiers that could reveal patient information. Business purpose classification distinguishes between emails supporting patient treatment, healthcare operations, payment activities, and other organizational functions. Different business purposes may trigger different retention requirements under various regulatory programs.
Retention Schedule Specifications
Minimum retention periods should reflect the longest applicable requirement from HIPAA email retention policy, state medical record laws, federal programs, and organizational needs. The policy should clearly state these periods for each email category and explain the basis for each requirement. Maximum retention limits help organizations manage storage costs and reduce litigation exposure by establishing when emails should be destroyed unless legal holds or other special circumstances require continued preservation. These limits should balance compliance needs with practical considerations. Exception procedures provide guidance for situations requiring deviation from standard retention schedules such as litigation holds, ongoing investigations, or patient access requests. These procedures should specify approval processes and documentation requirements for exceptions.
Storage and Archive Management Requirements
Security standards for archived emails must maintain the same level of PHI protection as active communications throughout the retention period. The policy should specify encryption requirements, access controls, and monitoring procedures for archived communications. Storage location specifications define where different types of email communications should be preserved including on-premises systems, cloud services, or hybrid approaches. These specifications should address data sovereignty, vendor requirements, and disaster recovery needs. Migration procedures ensure that archived emails remain accessible as technology systems change over time. The policy should address format preservation, system upgrades, and vendor transitions that could affect archived email accessibility.
Access Control and Retrieval Procedures
Authorization requirements define who can access archived email communications and under what circumstances. The policy should establish role-based permissions that limit access to personnel with legitimate business needs while maintaining audit trails. Search and retrieval protocols provide step-by-step procedures for locating archived emails during audits, legal discovery, or patient access requests. These protocols should specify search parameters, documentation requirements, and quality control measures. Emergency access procedures enable retrieval of archived communications during urgent situations when normal approval processes might delay patient care. These procedures should include alternative authorization methods and enhanced audit requirements.
Disposal and Destruction Standards
Secure deletion methods ensure that email content and metadata are completely removed when retention periods expire. The policy should specify approved destruction techniques that prevent unauthorized recovery of PHI from disposed communications. Certification requirements mandate documentation of email destruction activities including dates, methods used, and personnel responsible. These certifications support compliance demonstrations and help track disposal activities across the organization. Media destruction procedures address proper disposal of storage devices containing archived emails when equipment reaches end of life. A HIPAA email retention policy should specify physical destruction or certified wiping procedures that prevent PHI recovery.
Compliance Monitoring and Audit Support
Review schedules establish regular assessment of email retention practices to ensure continued compliance with policy requirements and changing regulations. These reviews should evaluate policy effectiveness, system performance, and staff compliance. Audit preparation procedures provide guidance for responding to regulatory reviews or legal discovery requests involving archived email communications. These procedures should include search protocols, production formats, and timeline management. Performance tracking helps organizations measure their success in meeting retention obligations while identifying areas needing improvement. Key metrics might include retention compliance rates, retrieval response times, and storage cost management.
Staff Training and Implementation Guidance
Training requirements specify education that personnel must receive about email retention obligations and their role in policy implementation. Training should cover classification procedures, retention schedules, and proper handling of archived communications. Implementation timelines provide realistic schedules for deploying new retention policies while allowing adequate time for staff training, system configuration, and process development. These timelines should consider organizational capacity and change management needs. Resource allocation addresses personnel, technology, and financial requirements for effective email retention policy implementation. The policy should specify roles and responsibilities while identifying budget needs for ongoing operations.
Legal and Regulatory Compliance Integration
Regulatory coordination ensures that a HIPAA email retention policy is adhered to, aligning with requirements from state laws, federal programs, and professional licensing boards. The policy should identify all applicable requirements and explain how conflicts are resolved. Legal hold procedures provide immediate preservation capabilities when litigation is anticipated or pending. These procedures should include notification processes, scope determination, and coordination with legal counsel to ensure comprehensive preservation. Update mechanisms ensure that retention policies remain current as regulations change or organizational needs evolve. A HIPAA email retention policy should specify review frequencies, approval processes, and communication procedures for policy modifications.