Explanation of benefits notifications are detailed summaries of healthcare claims processing that health plans send to members after receiving and adjudicating medical service claims from healthcare providers. These documents contain protected health information including patient names, dates of service, provider details, diagnostic codes, and payment information that falls under HIPAA privacy and security requirements. Healthcare providers, payers, and suppliers must understand how HIPAA regulations govern the creation, transmission, and storage of explanation of benefits communications to maintain compliance while serving their members effectively. Understanding the intersection of HIPAA requirements and explanation of benefits processes helps healthcare organizations avoid costly violations while maintaining transparent communication with patients about their healthcare coverage and claims.
Privacy Requirements for Explanation of Benefits Content
HIPAA privacy regulations establish specific requirements for how explanation of benefits documents can include, display, and protect patient information during all phases of the communication process. Health plans must ensure that explanation of benefits contain only the minimum necessary information required to inform patients about their claims processing while avoiding unnecessary disclosure of sensitive medical details. This requirement means that diagnosis codes, procedure descriptions, and provider notes should be limited to what patients need to understand their coverage and payment responsibilities.
The privacy rule permits health plans to include certain types of information in explanation of benefits without obtaining additional patient authorization, as these communications fall under permitted uses for payment and healthcare operations. Patient names, dates of service, provider names, and basic claim information can be included because they serve legitimate business purposes in helping patients understand their insurance coverage. Detailed clinical notes, mental health treatment specifics, or other sensitive medical information may require additional privacy protections or patient consent.
Explanation of benefits documents must include clear privacy notices that inform patients about how their protected health information is being used and their rights regarding this information. These notices should explain how patients can request restrictions on information use, file complaints about privacy practices, and access their complete medical records. Health plans must also provide contact information for privacy officers who can address patient concerns about their explanation of benefits communications.
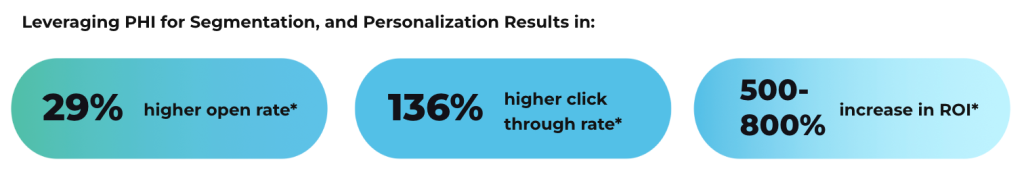
The minimum necessary standard requires health plans to evaluate whether all information included in explanation of benefits serves a legitimate purpose for patient understanding or claims administration. This evaluation should consider whether patients truly need access to specific diagnostic codes, provider credentials, or detailed procedure descriptions to understand their coverage. Regular review of explanation of benefits content helps ensure compliance with privacy requirements while maintaining useful communication with plan members.
Security Safeguards for Electronic Explanation of Benefits
Electronic transmission and storage of explanation of benefits requires implementation of administrative, physical, and technical safeguards to protect the protected health information contained within these documents. Administrative safeguards include appointing security officers responsible for explanation of benefits systems, conducting regular workforce training on privacy requirements, and establishing procedures for granting and revoking access to explanation of benefits databases. These safeguards help ensure that only authorized personnel can access patient information during explanation of benefits processing.
Physical safeguards protect the computer systems, equipment, and facilities where explanation of benefits are created, stored, and transmitted from unauthorized access or environmental hazards. Health plans must implement access controls for data centers, secure workstation configurations for staff accessing explanation of benefits systems, and media disposal procedures for devices containing patient information. Protections help prevent unauthorized individuals from accessing explanation of benefits data through physical security breaches.
Technical safeguards focus on access controls, audit logging, data integrity measures, and transmission security for explanation of benefits systems. Health plans must implement user authentication systems that verify the identity of individuals accessing explanation of benefits data, maintain detailed audit logs of all system activities, and use encryption to protect explanation of benefits during transmission and storage. Technical controls help detect and prevent unauthorized access to patient information.
Regular security assessments of explanation of benefits systems help identify vulnerabilities that could lead to data breaches or unauthorized disclosures. Health plans should conduct penetration testing, vulnerability scanning, and security audits of their explanation of benefits platforms to ensure that technical safeguards remain effective against evolving cyber threats. Documentation of these assessments demonstrates ongoing commitment to protecting patient information in explanation of benefits communications.
Patient Rights and Access to Explanation of Benefits
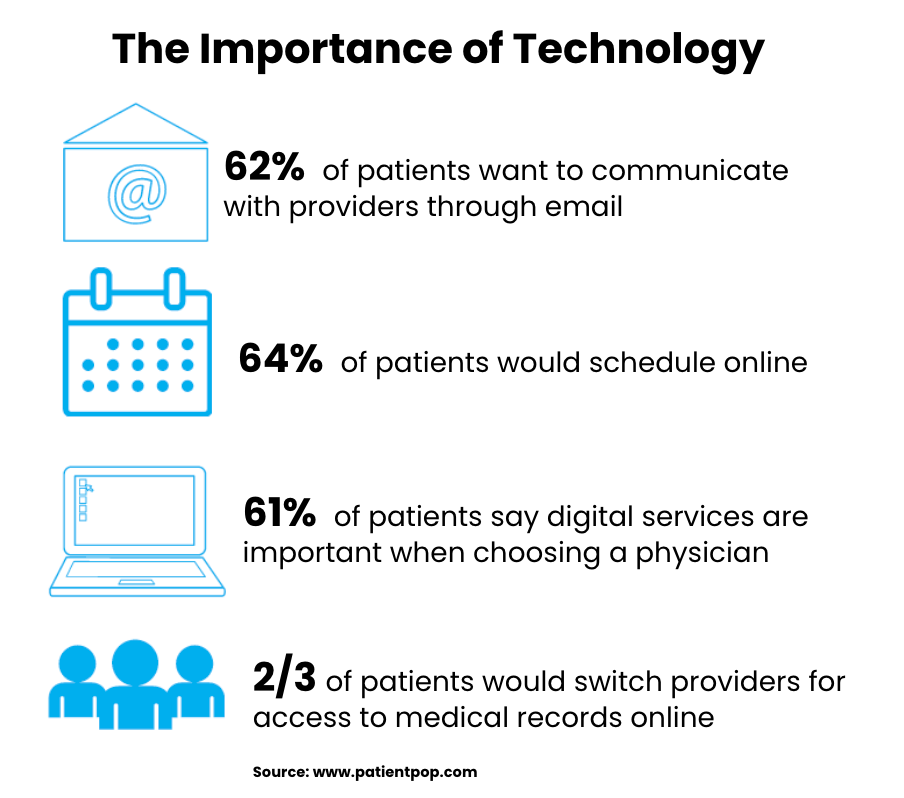
Patients have specific rights under HIPAA regarding their explanation of benefits, including the right to receive copies in accessible formats, request amendments to incorrect information, and control how these documents are delivered to them. Health plans must accommodate reasonable requests for explanation of benefits in alternative formats, such as large print, electronic delivery, or translation into other languages when patients have communication barriers. Accommodations help ensure that all patients can understand their coverage and claims processing regardless of their individual circumstances.
The right to request amendments applies when patients identify errors in their explanation of benefits, such as incorrect dates of service, wrong provider information, or inaccurate claim amounts. Health plans must have established procedures for handling these amendment requests, including timeframes for responding to patients and processes for investigating and correcting errors. When amendments are approved, health plans must notify patients and update their records accordingly.
Patients can designate how they prefer to receive explanation of benefits notifications, including requesting that documents be sent to alternative addresses for safety reasons or medical necessity. Health plans must honor these requests when they are reasonable and help protect patient privacy or safety. This flexibility allows patients to maintain control over their personal information while ensuring they receive important coverage information.
Access rights extend to requesting accounting of disclosures related to explanation of benefits information, allowing patients to understand who has received their protected health information and for what purposes. Health plans must maintain records of explanation of benefits disclosures and provide this information to patients upon request. These accounting requirements help patients monitor how their information is being shared and identify any unauthorized uses.
Disclosure Rules for Explanation of Benefits Information
HIPAA establishes specific rules governing when and how health plans can disclose explanation of benefits information to third parties, including healthcare providers, family members, and business partners. Disclosure for treatment purposes allows health plans to share relevant explanation of benefits information with healthcare providers who need this data to coordinate patient care or understand coverage limitations. These disclosures must be limited to information necessary for the specific treatment purpose.
Payment-related disclosures permit health plans to share explanation of benefits information with healthcare providers for billing and claims processing purposes. Providers may need access to explanation of benefits data to understand payment amounts, coverage decisions, and patient responsibility amounts. These disclosures help facilitate efficient payment processing while maintaining patient privacy protections.
Healthcare operations disclosures allow health plans to share explanation of benefits information for quality improvement activities, care coordination, and administrative functions that support patient care. These uses must serve legitimate business purposes and comply with minimum necessary standards. Health plans must evaluate whether proposed disclosures serve appropriate healthcare operations purposes before sharing explanation of benefits information.
Disclosure to family members or personal representatives requires either patient authorization or demonstration that the person has legal authority to act on behalf of the patient. Health plans cannot automatically share explanation of benefits information with spouses, adult children, or other family members without proper authorization. Emergency situations may provide exceptions to this requirement when immediate disclosure is necessary for patient safety or care coordination.
Business Associate Requirements for Explanation of Benefits Processing
Third-party vendors involved in explanation of benefits processing must operate as business associates under HIPAA and comply with specific privacy and security requirements when handling protected health information. Business associate agreements must clearly define how vendors will protect explanation of benefits data, limit its use to authorized purposes, and implement appropriate safeguards during processing activities. Agreements of this nature help ensure that outsourced explanation of benefits functions maintain the same privacy protections required of health plans.
Common business associates in explanation of benefits processing include printing companies, mailing services, electronic delivery platforms, and customer service providers. Each of these relationships requires careful evaluation of privacy and security risks, along with appropriate contractual protections. Health plans must verify that business associates have adequate security measures in place before allowing them to handle explanation of benefits information.
Business associates must implement their own administrative, physical, and technical safeguards for explanation of benefits data and ensure that any subcontractors also comply with HIPAA requirements. This includes providing security training to their workforce, maintaining audit logs of information access, and reporting security incidents to the health plan. Business associates also must return or destroy explanation of benefits information when their contracts end, unless retention is required for legal purposes.
Regular monitoring and oversight of business associate performance helps ensure ongoing compliance with HIPAA requirements for explanation of benefits processing. Health plans should conduct periodic audits of business associate security practices, review incident reports, and verify that contractual obligations are being met. This oversight helps identify potential compliance issues before they result in privacy violations or security breaches.
Compliance Monitoring and Breach Response
Healthcare organizations must establish comprehensive monitoring programs to ensure that explanation of benefits processing remains compliant with HIPAA requirements and identify potential issues before they result in violations. Regular audits should examine explanation of benefits content for appropriate privacy protections, verify that security safeguards are functioning correctly, and assess whether disclosure practices comply with regulatory requirements. Audits help demonstrate ongoing commitment to protecting patient information.
Incident response procedures specifically address explanation of benefits-related security breaches or privacy violations, including notification requirements and remediation steps. Health plans must have clear procedures for investigating potential breaches, determining whether notification is required, and implementing corrective actions to prevent future incidents. Training on incident response helps ensure that staff can recognize and respond appropriately to explanation of benefits security issues.
Documentation requirements include maintaining records of explanation of benefits policies, training activities, security assessments, and compliance monitoring efforts. This documentation helps demonstrate compliance efforts during regulatory investigations and supports continuous improvement of explanation of benefits processes. Health plans should retain documentation for required periods and ensure that records are complete and accessible when needed.
Staff training programs must address HIPAA requirements specific to explanation of benefits processing, including privacy obligations, security procedures, and appropriate handling of patient information. Training should be provided to all personnel involved in explanation of benefits creation, transmission, and storage, with regular updates to address regulatory changes and emerging threats. Competency assessments help verify that staff understand their responsibilities for protecting patient information in explanation of benefits communications.